Supplemental Material and Frequently Asked Questions
Supplementary Material
Cell Type
This section covers differences between cell types, technical considerations for using various cell types, experimental design considerations for various cell types, and cell culture.
Cell Culture
Cell culture refers to the growth of cells in a controlled, artificial environment. Isolated cells come from various biological sources, such as animals, plants, and fungi. Cell culture is typically divided into two groups: primary cell culture and immortalized cell lines. Cell lines are cells isolated from tissue and grown primarily in culture dishes or flasks until they reach confluency and must be subcultured. To answer today’s research questions, scientists will use both primary cells and established cell lines in assays such as the In-Cell Western.
Primary Cells
Primary cells are isolated directly from the tissue of interest and grown for a finite time in culture. These cells exhibit many of the same features as the tissue, such as morphology and expression profiles. The similarity between the tissue and the primary cells make primary cells useful for the study of various human diseases.
Immortalized Cell Lines
To maintain cell lines in culture, cells must be immortalized. Cells can be immortalized in a few ways.
-
The first is through spontaneous genetic changes, which give cells the ability to avoid senescence. This occurs in the case of cancer cells.
-
A second way cells can become immortalized is by introducing a viral gene, which confers the ability of cells to override the cell cycle. Genes for the large and small T antigens from Simian Virus 40 are frequently used for this purpose.
-
A third method to confer immortality to cells is the expression of genes such as Telomerase (hTERT), which abate senescence by extending the lengths of telomeres and allowing the cells to divide indefinitely.
Once the immortalized cell line is established, it can be grown using specific growth conditions and maintained for long periods of time. Some cell lines have been grown for decades, resulting in phenotypic and genotypic distinctions from the tissue they were originally isolated from. Even with these differences, scientists have relied on cell lines to answer important scientific questions for decades.
Cell Culture Systems
There are two systems for growing cells in culture: adherent cells and suspension cells.
-
Adherent: Cells are attached to a surface in a single layer (monolayer)
-
Suspension: Cells float freely in a medium
Cell culture conditions vary greatly depending on the cell type being grown, but there are some common conditions.
Common Conditions
There are some common considerations for all cells grown in culture, such as media, temperature, osmotic pressure, and pH (CO2). Whether you are growing cells in adherent or suspension culture, the optimal pH, temperature, and CO2 recommendations are identical for mammalian cells.
Table 1. The recommendations in this table are for mammalian cell culture, both adherent culture (cell lines and primary cells) and suspension culture.
| Condition | Recommendation |
|
Media |
+/- Serum |
|
pH |
7.4 |
|
Temperature |
36-37 °C |
|
CO2 |
4-10% |
Growth Culture Medium
The composition of the growth culture medium can vary depending on cell type. The primary components in a cell culture medium are:
|
|
The primary function of the medium components is to support and promote cell growth. Most components are required for all cell growth, independent of cell type. One factor which differs for different cell types is the presence of serum. High concentrations of serum can cause primary cells to change their cell type, a process known as differentiation. It can also promote the growth of certain cell types like fibroblasts more than others.
Primary cells also often require additional nutrients from traditional cell culture mediums, so each cell type may have a specialized media that is formulated which is low in serum or serum-free for their growth. Cell lines require less specialized mediums, but the presence and amount of serum can still vary depending on the type of cell line. Synthetic sera, which can have reduced variation between batches, are commercially available.
For more detailed recommendations for cell culture medium and culturing techniques for each cell line, you can refer to www.atcc.org.
Table 2. This table shows the components of growth culture medium and the purpose of those components.
|
Component |
Purpose |
|
Buffer |
Maintain pH, Source of sodium, potassium, carbon, nitrogen, hydrogen |
|
Amino Acids |
Promote cell growth |
|
Vitamins |
Promote cell growth |
|
Inorganic Salts |
Promote cell growth |
|
Polysaccharide (e.g., Glucose) |
Energy source |
|
Serum (e.g., Fetal Bovine Serum) |
Provides hormones, growth and adhesion factors, lipids, etc. for cell growth |
Experimental Design
Choosing a Cell Type
The cell type chosen for an In‑Cell Western Assay is critical for the success and relevance of the results. Both primary cells and immortalized cell lines can be used in an In‑Cell Western Assay, and there are specific considerations for each. If the appropriate cell type is chosen and antibodies are validated, the In‑Cell Western can provide accurate and relevant results to scientific questions.
Primary cells can be hard to grow and hard to transfect. For both primary cells and cell lines, different phenotypic and genotypic characteristics can directly affect the results of an assay. For example, if performing a cytotoxicity assay, cells that normally exhibit a high cell death rate may contribute background to the assay. Primary cells have a higher spontaneous cell death rate than cell lines, and therefore may not be optimal for cytotoxic assays. For cell viability assays, cell lines tend to have a higher metabolic rate.
Choosing a Plate Type
Different plates are recommended for adherent vs suspension cells for plate-based assays.
- For adherent cells, LI‑COR recommends a 96-well plate with a clear, flat bottom and black wells, such as the Greiner Bio-One CELLSTAR® Black μClear® Microplate, LI‑COR PN 926-19156 (8 pack) or 926-19157 (32 pack).
- For suspension cells, LI‑COR recommends growing cells in a 96-well U-bottom plate and transferring cells to Greiner Bio-One CELLSTAR® Black μClear® Microplate, LI‑COR PN 926-19156 (8 pack) or 926-19157 (32 pack), for imaging.
Considerations for Primary Cells
Primary cells can be difficult to culture in vitro. These are some general points to consider for culturing primary cells in vitro.
-
Prevent contamination: Upon initial isolation from tissue, cells should be treated to prevent contamination from bacteria or fungi.
-
Maintain less than 100% confluency: Cells should be grown in the appropriate medium with supplements until the desired confluency is attained, so that the primary cells can be subcultured. It is important not to allow primary cells to reach 100% confluency, because they can enter senescence (a process which inhibits cell division). Growing the primary cell culture past confluency may also lead to differentiation, where the cellular profile will be permanently altered and may ultimately affect how the cells will perform in an assay.
-
Limit length of time cells are exposed to trypsin: Primary cells are sensitive to trypsin, so adherent cells should not be exposed to trypsin for prolonged periods of time. Exposing primary cells to trypsin for prolonged periods of time can lead to cell death.
-
Maintain other growth conditions: Maintenance of the pH, temperature, and CO2 concentration is also very important, as primary cells are more sensitive to changes in these growth conditions than cell lines.
Considerations for Cell-Based Assays
Many factors will influence the outcome of a cell-based assay. The list below has important information to know regarding the cell type you choose for your experiments.
Obtaining Cells
Ideally, research would be conducted with authenticated, quality-controlled cell lines with a low passage number from a dependable biological resource center. Depending on the needs of your research and where you were able to obtain your cells, you may need to perform verification tests on your cell line to ensure the cells can be used for reliable and publishable research.
ATCC recommends “basic benchmark verification tests that can be employed by any lab and included in publication” (1), specifically covering the following subject areas.
-
Low passage number
-
Morphology check by microscope
-
Growth curve analysis
-
Species confirmation by isoenzyme analysis
-
Identity verification with STR profiling
-
Mycoplasma detection
Confirm Cell Line Identity After Subculturing in the Lab
Detailed discussion about confirming cell line identify is beyond the scope of this document, but ATCC provides useful articles on this topic. You can read more about how STR (short tandem repeat) profiling can contribute to quality and integrity of research using human cell lines in their article Searchable STR Database For Human Cell Lines (2). You can learn more about identifying mycoplasma contamination in cell cultures on their Mycoplasma Testing Service page (3).
Other Factors to Consider
Plate Treatments
-
Tissue-culture treated
-
Poly-L or Poly-D-Lysine
-
Gelatin
Cell Characteristics
-
Cell doubling time
-
Treatment conditions (such as serum starvation)
Cell Type References
1. ATCC. Cell Line Authentication - Why It Matters, (Acc. August 27, 2020).
2. ATCC. Searchable STR Database For Human Cell Lines, (Acc. August 27, 2020).
3. ATCC. Mycoplasma Testing Service, (Acc. August 27, 2020).
Other Resources
-
ATCC. Animal Cell Culture Guide, (Acc. August 27, 2020).
-
Zhanqiu, Y., Xiong, H. (2012). Culture Conditions and Types of Growth Media for Mammalian Cells. IntechOpen. https://doi.org/10.5772/52301
Cell Seeding
Cell seeding is the process of pipetting a specific number of cells into each well of a multiwell plate. This procedure has a marked effect on the assessed biological result of a treatment or condition. Multiple cell seeding concentrations should be tested to determine a concentration that will result in a level of confluency that generates significant well fluorescence at the time of imaging, without reaching over-confluency. Standardization of a cell seeding protocol for a particular treatment is important for reproducibility of the experiment and assay output.
Cell Seeding Factors
The following are important factors to consider when seeding adherent cells.
Plates
For cells that adhere strongly to the wells (e.g., A431, HEK293, CHO), we recommend regular tissue culture microplates with low auto-fluorescence, particularly those with black sides and clear well bottoms (e.g., Greiner CELLSTAR® 96 well plates, flat bottom black polystyrene wells). For adherent cells that could detach from wells during In-Cell Western Assay wash steps (e.g., NIH3T3), we recommend Poly-D-lysine coated 96-well microplates.
- For adherent cells, LI‑COR recommends a 96-well plate with a clear, flat bottom and black wells, such as the Greiner Bio-One CELLSTAR® Black μClear® Microplate, LI‑COR PN 926-19156 (8 pack) or 926-19157 (32 pack).
- For suspension cells, LI‑COR recommends growing cells in a 96-well U-bottom plate and transferring cells to Greiner Bio-One CELLSTAR® Black μClear® Microplate, LI‑COR PN 926-19156 (8 pack) or 926-19157 (32 pack), for imaging.
Cell Seeding Density
Typically, 5,000 to 40,000 cells are seeded per well (96-well plate). One to three days are required for cells to reach the appropriate confluency, depending on growth rate and cell size. The growth rate can be different at different cell densities. It is best to attempt to operate within the exponential growth phase for your cell line.
Seeding with lower cell numbers is recommended if you plan to culture for several days before use. Plates seeded with higher cell numbers will be ready to use earlier.
Confluence
In general, cells should be imaged at about 80 – 85% confluency while cells are in the log phase of growth and in a monolayer on the plate. However, the appropriate level of confluency depends on the cell line. Experimental conditions must be optimized for the cell type to determine the appropriate level of confluency required to achieve significant well fluorescence.
Generalized Protocol for Seeding Cells
-
Allow cells to grow in a T75 flask using standard tissue culture procedures until ~80% confluency is achieved.
-
Remove growth media and wash cells with sterile 1X PBS (RT).
-
Add 5 mL Trypsin-EDTA (Sigma) and incubate for 3 - 5 minutes at 37 °C and 5% CO2 to displace cells.
-
Neutralize trypsin by adding serum-containing growth media and pellet by centrifugation.
-
Remove supernatant and disrupt the cell pellet manually by hand-tapping the collection tube.
To maintain cell integrity, do not pipet or vortex during pellet disruption.
-
Dilute cells in complete media so that cells are at a concentration that is 5-fold the desired amount per well.
-
Manually mix the cell suspension thoroughly.
-
Under sterile conditions, dispense 200 μL of the cell suspension per well in a 96-well plate.
-
Incubate cells at 37 °C and monitor cell density until desired confluency is achieved.
Plate Loading Guides
To use the following plate loading guides, cut out the layout, then place the layout underneath the plate to make it easier to load samples in the correct wells. Plate layouts may also be created and printed from Empiria Studio® Software.
More Plate Loading guides are available at licor.com/plate-loading-guides.
Plate Layout for Loading Antibody Dilutions
The following plate layout can be used as a guide for loading antibody dilutions according to the Plate Layout shown in Single Antibody Titration Experiment.
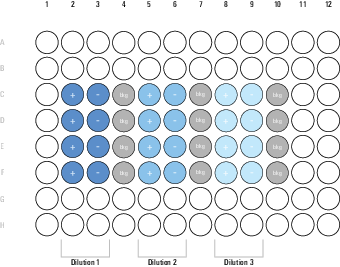
| Symbol | Well Type | Cells | Primary Antibody | Secondary Antibody | CellTag™ 700 Stain |
| bkg | Background | ||||
| + | Positive Control | ||||
| - | Negative Control |
Blank Plate Loading Guides
Label the following blank Loading Guides as needed for your In‑Cell Western Assay.
More Plate Loading guides are available at licor.com/plate-loading-guides.


Frequently Asked Questions
General
Question |
Answer |
| What is the In-Cell Western Assay? | The In‑Cell Western is a quantitative IF assay performed in microwell plates (optimized for 96- or 384-well formats). Data analysis is based on whole-well fluorescence quantification. The In-Cell Western Assay offers a convenient alternative to Western blotting and is a powerful platform for meaningful in situ analyses. |
| What is the difference with the antigen conformation between In‑Cell Western and Western blot? | In a Western blot, the antigen is in its denatured state, and the proteins are separated by size. In an In‑Cell Western, the antigen is in a more native conformation and the proteins are not separated by size. |
| What are the advantages of the In‑Cell Western vs IF? | An In‑Cell Western is a higher-throughput assay that allows for more samples to be quantified quickly in a plate based format. |
| What are the advantages of the In‑Cell Western vs ELISA? | The ELISA can only screen for 1 target, depends on the enzymatic reaction for chemiluminescence detection, requires a standard curve for quantification. |
| Why should I use the In‑Cell Western over flow cytometry? | The protocol and data analysis for the In‑Cell Western is more streamlined than the flow cytometry protocol. The In‑Cell Western lacks information on cell size and granularity. |
| What is the difference between In‑Cell Western and On-Cell Western? | In the In‑Cell Western, the cell is permeabilized to allow targeting proteins that are present inside of the cell. In an On-Cell Western, the cells are probed with antibodies prior to permeabilization (if your On-Cell Western requires permeabilization). |
| What are some common applications for this assay? | Some common examples of the use of the In‑Cell Western are: studies of signaling pathways, detection of post-translational modifications such as protein phosphorylation, timing/kinetics of signal transduction, IC50 determination, GPCR activation, protein accumulation and inhibition, viral titer/load, apoptosis, cell surface proteins and receptor internalization, RNAi efficacy, cell proliferation assays, and targeted therapeutic development. |
| What specific reagents do I need to get started? | Black walled, flat bottom plates of 96 or 384 wells, fixation and permeabilization reagents (typically PFA and Triton™ X-100), blocking buffer (Intercept® Blocking Buffer), primary antibody, secondary antibody (IRDye® 800CW), and normalizing stain (CellTag™ 700 Stain or CellTag 520 Stain). |
Assay Development
Question |
Answer |
| How many targets can each well have? | See licor.com/DetectionReference for more information about detection channel capabilities on Odyssey Family imagers. |
| What time point should I use for this assay? | The time point for treatment will depend on previous experimental data that indicates the time point that produces the desired result. |
| Can I perform In‑Cell Western on organoids? | Yes, the In‑Cell Western is compatiable with organoids as well as differentiated cells. |
| What steps and reagents need to be optimized? | Every step of any assay can be optimized, the steps and reagents that are commonly optimized for the In‑Cell Western are the cell seeding, blocking buffer evaluation, fixation/permeabilization, and primary antibody concentration. |
| In what order should the steps be optimized? | The preferred order in which steps will be optimized will be dependent on the particular assay but as a rule of thumb, we typically develop the protocol in the order each step takes place. |
| What are some common challenges with technique? | The most common challenges with the technique for the In‑Cell Western is pipetting too far into the well and removing the cells, shaking the plate too fast, and not optimizing reagents/conditions. |
| Can the In‑Cell Western be performed with bacteria and yeast? | The In‑Cell Western can be performed on bacteria and yeast, however, there are a few additional modifications to the protocol to account for the presence of a cell wall. |
| Can I use the In‑Cell Western for secreted proteins? | No, the In‑Cell Western cannot be used for secreted proteins as they are washed away during the wash steps. |
| What type of controls do I need? | When optimizing the reagent conditions, include a secondary antibody background control (no primary antibody or CellTag™ Stain), a negative control, and a positive control. |
| What should I do if I don't see a difference between my positive and negative controls? | Confirm that there are different expression levels between the positive and negative control in another assay, such as a Western blot. Proceed with further protocol development. |
| How do I prevent my cells from clumping in the center of the well? | Homogenize the cells carefully before seeding and incubate for 1 hour at room temperature before placing in the incubator. Prevent cell over growth. |
| Can I strip a plate and reprobe for other targets? | Stripping and reprobing of plates has not been validated by LICORbio. |
Cell Seeding
Question |
Answer |
| How do you handle suspension cells? | Grow and treat suspension cells in the appropriate growth conditions, and add them in the flat bottom plate at the time of fixation. In some cases, the treatment can be performed with the cells already seeded in the flat bottom plate. |
| What number of cells should my plate have? | The number of cells to load in each well varies based on the cell line. Typically, 5,000 to 40,000 cells are seeded per well. One to three days are required for cells to reach the appropriate confluency, depending on growth rate and cell size. Seeding with lower cell numbers is recommended if you plan to culture for several days before use. Choose a cell seeding amount where the signal is in the linear range. |
| Can I use the In‑Cell Western for primary/differentiated cell cultures? | Yes, when working with primary cell lines prevent contamination, maintain less than 100% confluency, limit the length of times the cells are exposed to trypsin, and maintain growth conditions (such as pH, temperature, etc.). |
| How many cells do I need to have per well to see signal? | The number of cells is only one contributing factor to the signal in the well. Cell line, antibody specificity, protein expression level, and other factors will contribute to the signal. Therefore, those conditions need to be determined empirically. |
Fixation and Permeabilization
Question |
Answer |
| What are the most common fixation and permeabilization reagents? | The most common fixation and permeabilization reagents are 3.7% formaldehyde and 0.1% Triton™ X-100, respectively. |
| When optimizing permeabilization and blocking: which concentration of primary antibody do I have to use? | Use the recommended primary antibody concentration as indicated on the data sheet from the manufacturer for IF. |
Primary Antibody
Question |
Answer |
| How do I select which primary antibody to use? | Choose a primary antibody that is validated for IF/ICC/IHC. You can reach out to LICORbio for suggestions of primary antibodies based on published data, and validate the antibodies using Western blot and IHC. |
| How do I validate my primary antibody? | Confirm antibody specificity through Western blot looking for the size of your target protein, absence of non-specific bands, and appropriate expression levels of target protein in positive and negative controls. Use IHC/IF to confirm that the protein localizes to the appropriate cellular location and to determine the concentration of the primary antibody. |
Normalization
Question |
Answer |
| What are the options for normalization? | The signal for the target protein can be normalized against a cell stain, such as CellTag™ 520 Stain or CellTag 700 Stain. Post translational modifications can be analyzed with the In‑Cell Western. The phopshorylated target is normalized against the pan or total protein signal. |
| How should I normalize in an On-Cell Western Assay? | The target signal can be normalized against a cell stain (such as CellTag 520 Stain or CellTag 700 Stain) after the plate has been imaged for the primary target of interest, because the cells will need to be permeabilized at this point. |
| Can I use Revert for In‑Cell Western normalization? | No, neither Revert™ 520 Total Protein Stain nor Revert 700 Total Protein Stain can be used for normalization in an In‑Cell Western Assay. We recommend using a total cell stain, such as CellTag 520 Stain or CellTag 700 Stain. |
Wash Step in the Protocol
Question |
Answer |
| How do I wash the cells? | The cells can be washed manually by gently pipetting the wash solution down the inner wall of the plate or using an automated plate washer. |
| Can I use an automated plate washer? | Yes, when using an automated plate washer ensure that the pressure is low and does not wash away the cell from the well. |
| Is it better to remove solution from the well by pipetting or by vacuum aspiration? | Both methods have their advantages and disadvantages and should be chosen based on your research needs. |
Plates
Question |
Answer |
| What plates should I use? |
Use black walled, clear flat bottom plates.
|
| Do I have to use the recommended Greiner plates? | No, you do not have to use the Greiner plates. Plates with any number of wells can be used. If you choose to use another plate, be sure that the plate has a clear bottom and has black walls. The focus offset must be optimized by scanning the plates at several focus offset settings and choosing the focus offset that yields the highest signal-to-noise ratio. |
| Can I use treated/coated plates? | For adherent cells that could detach from wells during In‑Cell Western Assay wash steps (e.g., NIH3T3), we recommend Poly-D-lysine coated multiwell plates. |
Imaging Microwell Plates
Question |
Answer |
| How do I determine the correct focus offset for my plate? |
The Multiwell Plate imaging workflows in LI‑COR® Acquisition Software are setup with recommended imaging parameters for LICORbio imagers. For more information, see the LI‑COR Acquisition Software Help (licor.com/AcquisitionQuickStart) or the Operator's Manual for your imager. Be sure to determine the correct focus offset if you are not using the recommended plates (see Plates) with your Odyssey Imager. To manually determine a focus offset, scan the plate at various focus offsets and choose the focus offset that yields the highest signal for the target protein. Most plates have a focus offset between 3.5 - 4.0 mm. |
| How would I know from looking at an image that the focus offset is incorrect? | If the focus offset is incorrect, the image will appear fuzzy and there will be a ring of signal on the outer edges of the wells. |
| Why is there a halo on the edge of the wells in my image? | There could be several causes for this effect. The focus offset may not be correct and the plate is being scanned out of focus. The cells may be accumulating at the edges of the well. If white or transparent walled plates are used, then the plate is exhibiting autofluorescence. |
| What settings should I use to acquire images? | The Multiwell Plate imaging workflows in LI‑COR Acquisition Software are setup with recommended imaging parameters for LICORbio imagers and the recommended plates (see Plates). For more information, see the LI‑COR Acquisition Software Help (licor.com/AcquisitionQuickStart) or the Operator's Manual for your imager. |
| I see cells under a microscope but no signal on the plate what does that mean? | Confirm that the plate is imaged using the correct focus offset. If the focus offset is correct, optimize reagents and conditions. Include CellTag™ 700 Stain as a positive control. |
| How much liquid should be in the plate when I image? | Plates should be inverted and blotted dry and then sealed prior to imaging and storage. |
| Why is my dilution series (i.e., cell seeding density, drug treatment, etc.) not giving a linear signal? | The protocol likely needs to be further developed to ensure you are operating within the linear range of the assay. See Experiment Linearity for more information about cell stain linearity. Empiria Studio® has a Cell Stain Linearity workflow and a Preset Plate Template for analyzing a Cell Stain Linearity experiment. See the Empiria Studio Software Help (licor.com/AnalysisQuickStart) for more information about using Plate Templates for analylsis. |
Miscellaneous
Question |
Answer |
| How long can I store the plates after completion of the In‑Cell Western experiment? | Store at 4 °C and sealed in a light tight container for several weeks. |
| How can I know if my assay is properly developed? |
The Z'-factor can confirm robustness of the assay. See Z’-Factor Determination for more information about the Z'-factor. Empiria Studio® Software has a Z'-Factor Determination workflow and a Preset Plate Template for analyzing a Z'-factor experiment. See the Empiria Studio Software Help (licor.com/AnalysisQuickStart) for more information about using Plate Templates for analylsis. |
| How do I analyze the data? | See the Empiria Studio Software Help (licor.com/AnalysisQuickStart) for more information about using Plate Templates for analylsis. |
| Has In-Cell Western data been published? | Yes, there are >1000 publications that use the In‑Cell Western Assay. |
| What could lead to speckling on the plate? | Lint or dust contamination can come from the working surface or scan area and can lead to speckling. |
| What could lead to speckling in the wells? | Speckling could be caused by antibody aggregation. Spin down the antibody tube and sterile filter the primary and secondary antibody solution. |
| What is a good stopping point in the In‑Cell Western protocol? | The fixation step may work as a stopping point for your protocol. |
| Can the plates be heated to dryness or taken from the fridge immediately before imaging? | Temperature can affect fluorescence and reading at inconsistent temperatures can cause results to vary. |