Therapeutic Response Applications
Optical imaging is ideal for observing and characterizing delivery of your therapeutic in live animals and excised organs. Optical modalities include direct detection using near-infrared (NIR) fluorescent dyes and indirect detection using bioluminescence or NIR fluorescent proteins.
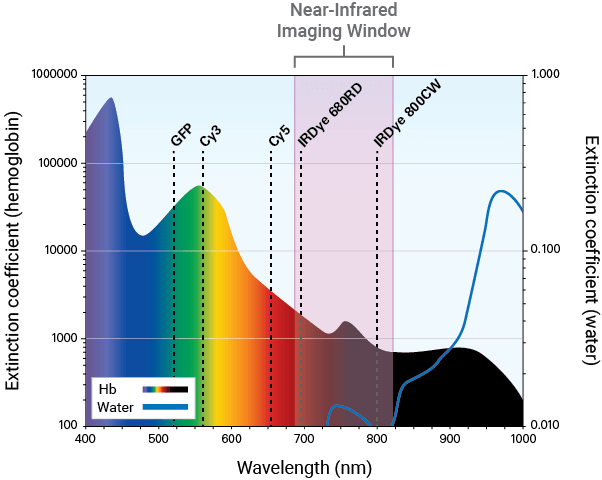
NIR fluorescence detection (680 nm to 820 nm) is a sensitive and efficient modality for in vivo and ex vivo studies. It can be used to illuminate features, such as tumors, deep within the body.
Near-Infrared Imaging for Therapeutic Development
NIR imaging aids in visualizing and monitoring the response to your therapeutic. Developing a dye-labeled therapeutic involves several validation steps. Characterization of therapeutics should be done in vitro to confirm specificity to the target before moving to animal studies, as in vivo studies can be very costly.
Comprehensive in vitro analysis of your therapeutic can be performed using an Odyssey® Imaging System, such as the Odyssey CLx. Biological characterization of your dye-labeled therapeutic in vivo and ex vivo can follow using the Pearl® Trilogy Small Animal Imaging System.
Detection in the NIR spectrum is ideal for evaluating therapeutic response and provides several advantages.
Sensitivity
Autofluorescence from animal tissue is significantly lower in the NIR region. This renders tissue virtually transparent and allows for high signal-to-noise ratios and detection of your targets using dye-labeled therapeutics.
Throughput
In contrast to other imaging modalities, images can be captured within seconds, allowing you to rapidly and repeatedly screen mice.
Cost
Compared to other modalities, NIR imaging is more economical.
Measuring Therapeutic Activity with Transfected Cells
The molecular mechanisms behind the activity of your therapeutic can be measured using transfected cells or transgenic animals expressing either the luciferase gene (i.e., bioluminescence) or fluorescent proteins like near-infrared fluorescent protein (iRFP) (i.e., fluorescence). With specific promotors, these genes can be used to monitor levels of expression—either constitutive or inducible—in an animal within the context of the disease being studied.
Using Bioluminescent Proteins for Detection and Imaging
Bioluminescence is generally used for cell tracking and gene expression studies. Renilla luciferase and Firefly luciferase are commonly used enzymes in bioluminescence research. These enzymes oxidize the bioluminescent substrate luciferin, which results in an emission of light that can then be detected and measured. Cells that constitutively express the luciferase gene can be injected into a mouse and followed over time for cellular distribution after luciferin administration. Likewise, cells that express the luciferase gene under an inducible promotor can be monitored through various treatments to assess how the levels of expression change.
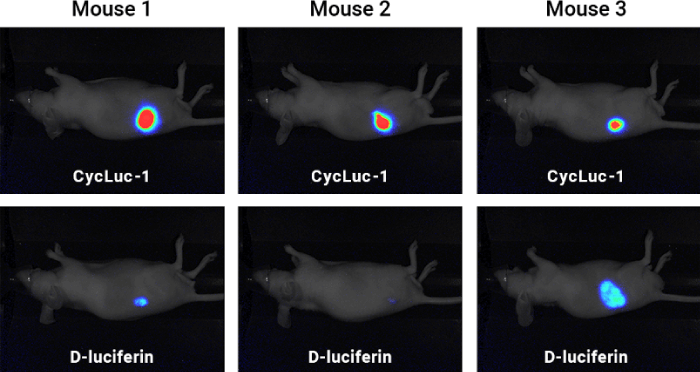
Bioluminescence can be detected using an imager like the Pearl Trilogy Small Animal Imaging System for in vivo molecular activity measurements. For example, a study compared D-luciferin and Cycluc-1 to determine which was a more robust luciferin substrate (Figure 1). A cell-construct was implanted in mice, and xenografts were established. Upon the injection of substrate, the Pearl Trilogy Imager was used to measure light production, and results suggested that Cycluc-1 was a more robust substrate than D-luciferin.
Fluorescent Protein Expression and Detection
NIR fluorescent protein expression can be used to measure cell tracking and tumor progression in response to your therapeutic. Unlike bioluminescence, iRFP does not require the addition of a substrate for visualization but simply needs to be excited using a light source. The emitted light is then detected by an imaging system such as the Odyssey CLx Imaging System or Pearl Trilogy.
Detecting Cancer Cell Lines and Monitoring Tumor Growth with iRFP
For in vivo therapeutic response measurements, fluorescent protein expression can be detected using the Pearl Trilogy. In the study below, iRFP was used to detect the prostate cancer cell line 22Rv1 and monitor tumor growth in mice (Figure 2). Images were then captured with NIR fluorescence and bioluminescence on the Pearl Trilogy Imager.
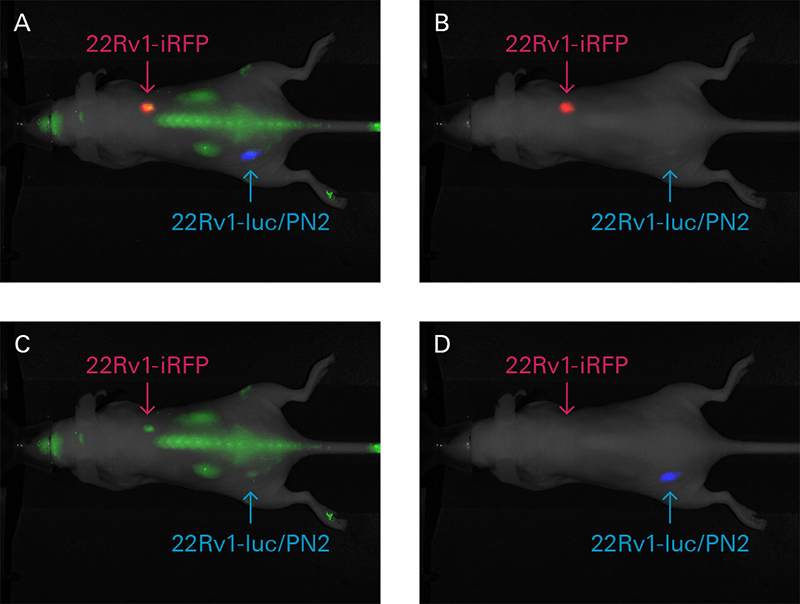
Figure 2. Mouse xenografts from iRFP-22Rv1 or 22Rv1-luc/PN2 constitutively expressing cells were prepared. Luciferase-expressing cells were implanted subcutaneous into the lower left hind flank, and iRFP-expressing cells were implanted subcutaneous into the right shoulder. IRDye® 800CW EGF (green) was administered intravenously (1 nmole) to detect EGF expression in vivo. Images of iRFP (red) and IRDye 800CW EGF expression were captured 48 h after injection, while bioluminescence (blue) was captured 10 minutes after D-luciferin injection. Individual images were captured in the 700 nm (B), 800 nm (C), and bioluminescence channels (D). (A) All three channels are displayed in a composite image. 22Rv1-luc/PN2 construct provided courtesy of Dr. Michael Henry, University of Iowa (see Drake, J.M., et al. for construct details).1
Detecting iRFP in Small Cell Populations and Monitoring Tumor Growth
Reporter proteins are frequently used to study the regulation of gene expression in vitro and in vivo. A reporter gene is placed under regulatory control of a promoter of interest. Upon expression, it produces either a luminescent or fluorescent protein that can be used to detect protein localization or measure expression levels. There are several fluorescent reporter proteins that are commonly used; iRFP713, for instance, is an NIR fluorescent protein with high intracellular stability and brightness compared to other phytochrome-derived fluorescent proteins. It is non-toxic in mammalian cells. Upon excitation of light at 690 nm, a signal is emitted at 713 nm that can be detected and measured using an imager.2
The expression of an infrared fluorescent protein such as iRFP713 alleviates some of the issues seen with visible reporter proteins like GFP.3 NIR wavelengths allow for deeper tissue penetration of excitation light as well as minimal background autofluorescence of cells and tissues. iRFP713’s properties and the benefits of NIR wavelengths make iRFP713 a valuable tool for researchers wanting to study protein expression in both cells and live animals.
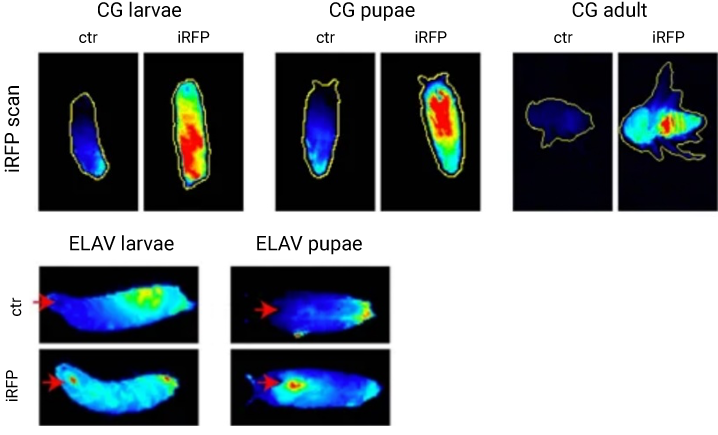
A study by Hock et al., detected in vivo iRFP713 expression and confirmed its sensitivity in a Drosophila model with NIR fluorescence on an Odyssey Imager (Figure 3). iRFP713 was then used to monitor tumor progression in a genetically engineered mouse model (GEMM) in vivo with the Pearl Trilogy Imager (Figure 4).
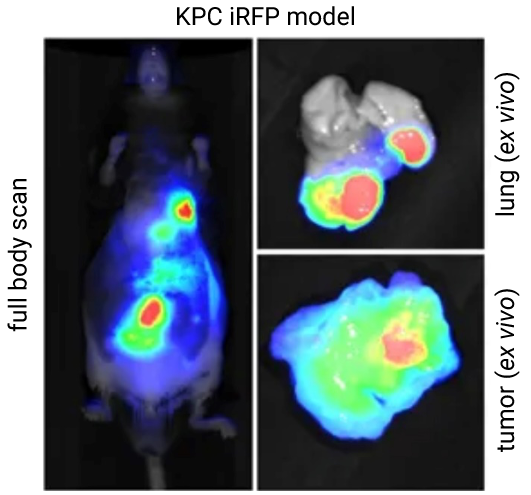
Tracking Therapeutic Response
The ability to visually track disease response to your therapeutic is essential to establishing therapeutic efficacy. For example, a probe labeled with a fluorescent dye or iRFP can be used to target a tumor. After a therapeutic has been administered, response to the therapeutic can be monitored over time.
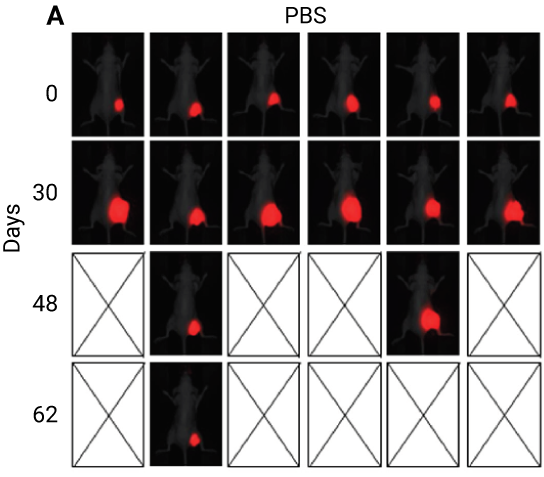
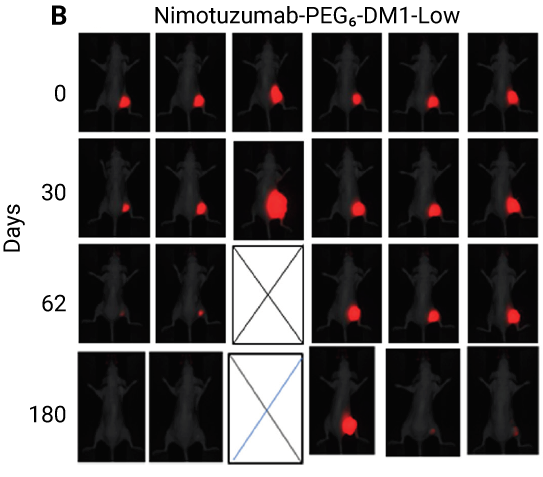
In the study below, nimotuzumab (an anti-EGFR antibody) was conjugated to PEGylated-maytansine (PEG6-DM1) to reduce the effect of acquired resistance. Mice bearing colorectal cancer tumors transduced to iRFP702 were treated using various means, including conjugated nimotuzumab. Images captured using the Pearl Imager (Figure 5) show that conjugating nimotuzumab to PEGylated-maytansine (PEG6-DM1) can improve its efficacy.
Bone Imaging
What is Bone Imaging?
Bone imaging is commonly used to provide anatomical landmarks to localize an area of tumor or disease. For instance, skeletal structures can be visualized using a dye-labeled bone targeting compound, while a second tumor-targeting agent labeled with a spectrally distinct dye, can help localize the tumor to bone anatomy. The two images can be overlaid to better define the location of the tumor or disease. Additionally, bone imaging in bone remodeling studies can detect bone growth or bone loss due to disease.
Bone Imaging with Near-Infrared Fluorescence
BoneTag™ Optical Probes are calcium-chelating compounds conjugated to a NIR dye to enable detection of bone mineralization, growth, and morphological changes. BoneTag Optical Probes are available with the following NIR dyes: IRDye 800CW (IRDye 800CW BoneTag) and IRDye 680RD (IRDye 680RD BoneTag). An IRDye 680RD BoneTag probe can be imaged at the same time as another IRDye 800CW labeled targeting agent using the two-color imaging capabilities of the Pearl Trilogy Imaging System.
"…Investigators in the Vanderbilt Center for Bone Biology are using that probe [developed in Manning lab] to look at TGF‑beta in bone, and in and around tumors that may be in the bone, and that's been very fruitful with the Pearl."
Resources
References
- Drake, J.M., Gabriel, C.L., and Henry, M.D. (2005). Clin Exp Metastasis, 22(8), pp 674-84. DOI: 10.1007/s10585-006-9011-4.
- Filonov, G.S., et al. (2011). Nature Biotechnology, 29(8), 757–761. DOI: 10.1038/nbt.1918.
- Hock, A.K., et al. (2017). Scientific Reports, 7(1), 1837. DOI: 10.1038/s41598-017-01741-0.
- Hartimath, S.V., et al. (2019). Oncotarget, 10, 1031-1044. DOI: 10.18632/oncotarget.26613.
