Do you know what factors affect normalization? Routine steps in the Western blotting process such as sample preparation, sample loading, and the detection of multiple proteins can introduce unwanted variability. You should plan to reduce error in every step of the Western blotting process. Without planning, you might get pseudo-quantitative results that don’t reflect the biology of your samples.
Sample Preparation

The way you prepare your samples can significantly change the results of your experiment. Even small changes in plating, cell lysis, reagent volume, and other technical details can have a surprising impact.
For example, how you lyse your cells affects protein extraction, solubilization, and modification status. The insoluble fraction may retain relevant proteins, affecting your quantitative analysis. Some experimental treatments shift fractions between soluble and insoluble.
For these reasons, it’s important to be consistent when preparing your samples. It’s also good practice to estimate the total protein concentration of each sample after preparation. Bradford, BCA, and Lowry assays are widely used to estimate the total protein concentration. Then it is possible to adjust gel loading to the estimated protein concentration.
Sample Loading
Overloaded gels create problems. Although strong bands may appear similar, the bands could be saturating either the membrane capacity or the dynamic range of detection. To avoid saturation and inaccurate results, run a standard curve with two-fold serial dilutions of cell lysate. You can then identify the linear range for each target protein.
Detection of Multiple Proteins
You may need to detect multiple proteins to compare relative protein levels, especially if you are using a housekeeping protein or signaling protein to normalize. Stripping and reprobing is often used to compare different proteins on the same blot, but it can introduce error. Leftover antibodies from incomplete stripping result in artifacts. Overly harsh stripping may result in a loss of sample proteins from the membrane. If, however, you use near-infrared fluorescent detection, there’s no need to strip and reprobe.
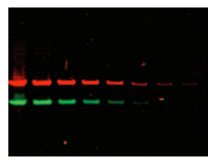
Multiplexing is when you detect two different proteins with spectrally-distinct secondary antibodies. Multiplexing is convenient and saves time. It is also more accurate than stripping and reprobing, because no artifacts are introduced, and there’s no possibility for protein loss.
With multiplexing, you can use co-migrating proteins, as well as easily identify antibody cross-reactivity.
For more details about factors that affect normalization, check out the full review article: Western Blot Normalization: Challenges and Considerations for Quantitative Analysis
Powered by Froala Editor