An effective internal loading control has a linear, proportional response, meaning the signal intensity of the internal control should accurately reflect sample concentration and abundance of loading control over a wide range. If your loading control does not meet the requirement of a linear response, it affects your accuracy and reproducibility.
Saturation limits the accuracy of normalization, especially if you’re using a housekeeping protein. Housekeeping proteins are often highly abundant in samples, which can lead to strong, saturated signals. Let’s look at what saturation is and where it can happen.
What Is Saturation?
Saturation is when strong signals don’t accurately reflect protein levels. It can come from your membrane, your detection chemistry, and the way you image your blot. Saturated bands are deceptive (Fig. 1). They hide actual variation in protein levels and underestimate the amount of protein present. The similar apparent intensities of saturated bands may lead you to think your protein levels are equal.
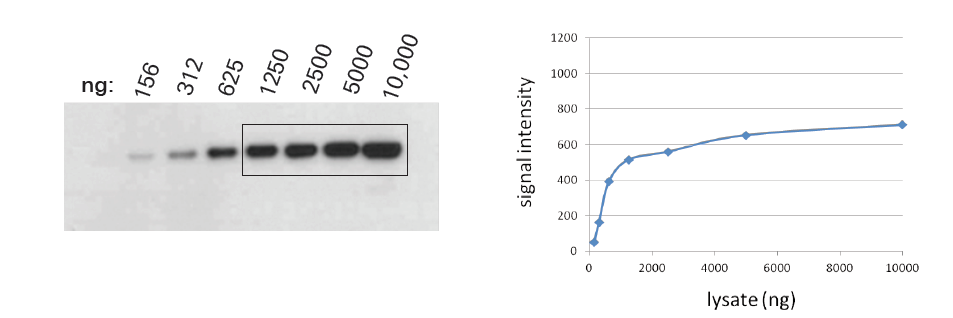
Membrane Saturation
If you have overloaded the samples on your gel, that problem does not go away once you transfer to the membrane. You may lose protein while transferring to the membrane, if overloaded samples exceed membrane capacity. In addition, highly abundant proteins might stack on top of each other.
When primary antibodies can only access the top layer of the protein stack, they can’t detect the rest of the proteins. This leads to underestimation of strong signals, hurting accurate quantitation. How can you prevent membrane overloading?
It’s best to run a dilution series to determine the upper limit of how much sample you should be loading on your gel. Membrane overloading is tricky to avoid, because different proteins generally have different upper limits in the same sample. Because it arises from the binding chemistry of proteins and blotting membranes, membrane saturation can happen with any detection chemistry or imaging method.
Detection Chemistry Saturation
When internal loading control bands are detected outside the linear range of detection, increases in protein level won’t produce a proportional increase in signal intensity. For accurate normalization, both the internal loading control and the target must be detected within the linear range of the method used. The type of detection chemistry you use affects the linear range of detection for your sample proteins.
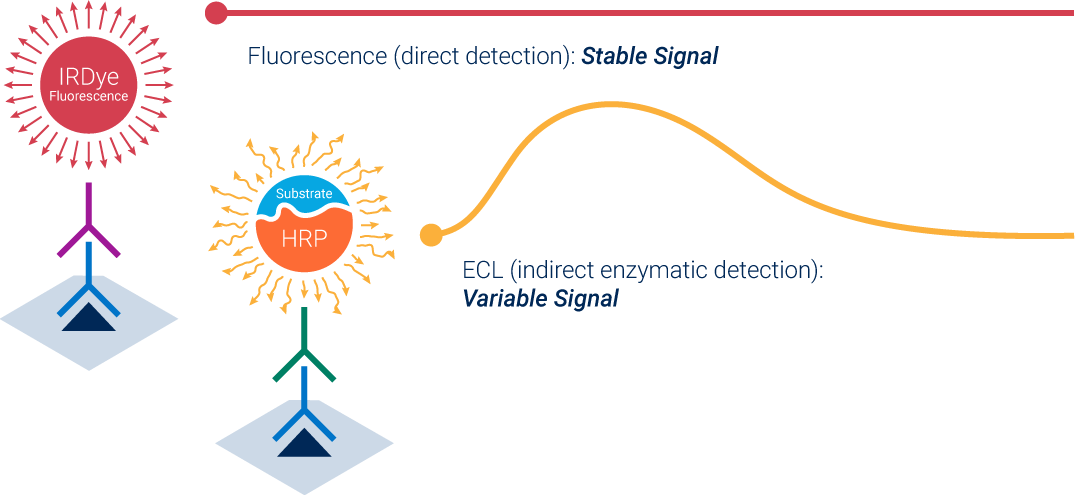
Enhanced chemiluminescence (ECL) is an indirect, enzymatic method. Secondary antibodies are labeled with horseradish peroxidase (HRP) as an enzymatic reporter. The enzyme produces light after you apply substrate and produces an unstable, time-dependent signal. Because these signals are the result of the kinetics of an enzymatic reaction, the signal doesn’t reflect its protein abundance. Saturation is likely with ECL, because it amplifies signals.
Fluorescence, on the other hand, is direct detection. Fluorophores label secondary antibodies and then generate stable signals. This type of detection chemistry doesn’t depend on enzyme kinetics, so fluorescent detection is more reproducible than ECL detection. Fluorescence is also less likely to saturate, because the signals are directly proportional to the amount of protein.
How to Avoid Saturation
How can you prevent detection chemistry saturation? The simplest way is to use fluorescent detection instead of ECL, because fluorescence is much less likely to saturate. If you must use chemiluminescence, then consider transitioning to digital imaging. If you have questions about fluorescence, saturation, or detection chemistry, then please feel free to contact us for more information. We'd love the opportunity to show you and your team the benefits of near-infrared fluorescence firsthand.
Powered by Froala Editor