Why Characterize Aquatic Carbon?
Characterizing carbon is key to unraveling its role in and impact on aquatic environments. Measurements of carbon provide valuable insights into carbon fluxes, cycles, and exchanges as well as ecosystem production, anthropogenic emissions, and the global carbon budget.
In particular, carbon measurements help researchers better understand and assess ocean acidification—a hazardous consequence of rising CO2 levels in the atmosphere—and its ramifications for aquatic life. This can ultimately contribute to the development of climate change mitigation strategies, conservation efforts, and sustainable management practices.
How is Aquatic Carbon Measured?
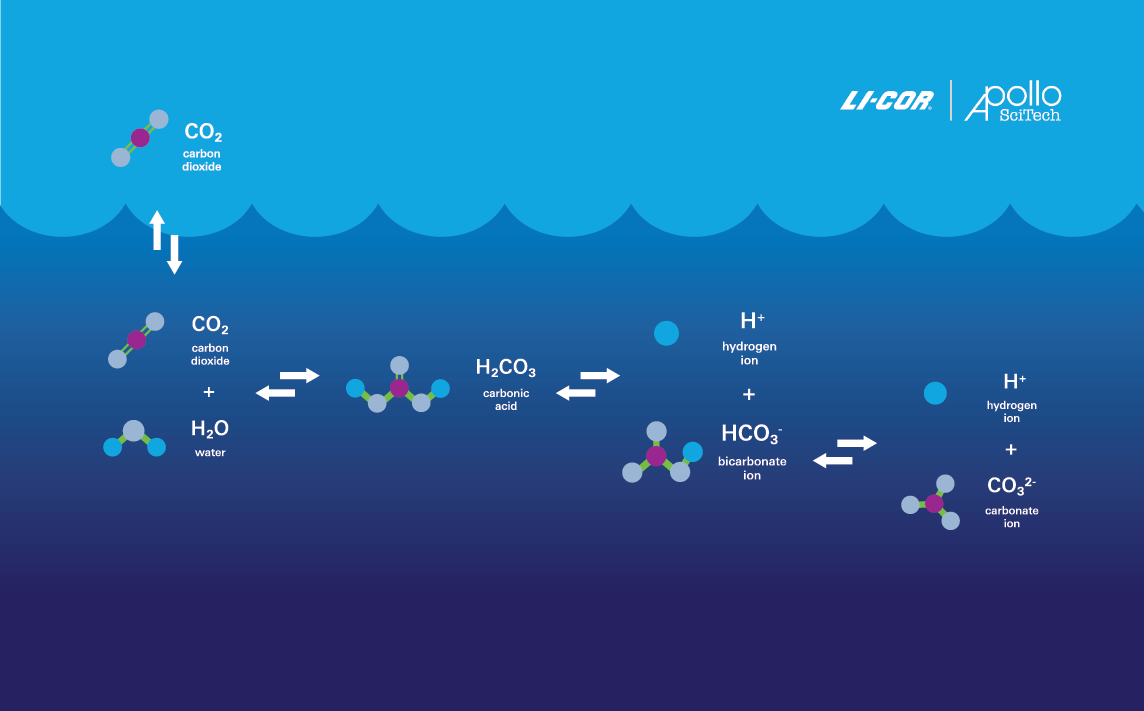
Because CO2 is the dominant product of organic carbon degradation across nearly all aquatic environments, its fluctuations are a key indicator of net ecosystem metabolism (Hopkinson, 1985; Smith and Hollibaugh, 1993). As such, it is essential to measure parameters that define the CO2 system. Today, there are four readily measurable parameters—all of which can be determined using Apollo SciTech aquatic instruments, which were acquired by LI-COR Environmental in 2023.
- The Dissolved Inorganic Carbon Analyzer measures dissolved inorganic carbon (DIC), or the concentration of CO2 and other inorganic carbon species dissolved in water.
- The Underway pCO2 System measures partial pressure of CO2 (pCO2), or the concentration of CO2 gas in water.
- The Spectrophotometric Seawater pH Analyzer measures pH, or the acidity (concentration of H+) or alkalinity (concentration of OH-) of water.
- The Total Alkalinity Titrator measures total alkalinity, or water’s capacity to neutralize acids.
Who Should Measure Aquatic Carbon?
Aquatic carbon measurements can benefit researchers in a wide variety of disciplines. The following research fields or interests commonly take these measurements to better understand biological processes, climate change, ecology, ocean acidification, and more.
Oceanography
Marine biology
Limnology
Fishery management
Shellfish ecology and production
Wetland, estuary, or open ocean carbon cycle research
Water-atmosphere or water-sediment exchange of CO2
Carbon sequestration research
Oceanography
Marine biology
Limnology
Fishery management
Shellfish ecology and production
Wetland, estuary, or open ocean carbon cycle research
Water-atmosphere or water-sediment exchange of CO2
Carbon sequestration research
References
Hopkinson, C.S. (1985). Shallow-water benthic and pelagic metabolism: Marine Biology, 87(1), pp.19–32. doi:https://doi.org/10.1007/bf00397002.
Smith, S.V. and Hollibaugh, J.T. (1993). Coastal metabolism and the oceanic organic carbon balance. Reviews of Geophysics, [online] 31(1), pp.75–89. doi:https://doi.org/10.1029/92rg02584.
Dive deeper into your research.
Get a Quote