Safety
Safety Considerations
Laser Safety
The Center for Devices and Radiological Health (CDRH) was established in October 1982, by the U.S. Food and Drug Administration (FDA) to protect the public health in the fields of medical devices and radiological health.
Manufacturers of products subject to performance standards under the Radiation Control for Health and Safety Act of 1968 are required to furnish various reports to the CDRH.
The Odyssey XF Imager is certified as a Class I laser product. This means that hazardous laser radiation is not emitted outside the instrument. Radiation emitted inside the Odyssey XF Imager is confined within protective housings and external covers. A series of safety interlocks ensures that the laser beam cannot escape during any phase of user operation.
One laser emits at 785nm and the other one at 685-690nm. The 785nm laser has a peak power rating of 1.2 watt. The 685-690nm laser has a peak power rating of 0.5 watt. The lasers emit visible and invisible laser radiation - direct exposure to either beam may cause eye damage. Laser radiation from each laser uniformly illuminates the target area only, which is marked in the imaging tray (Figure 68) that fits in the imaging drawer. There is no emission of laser radiation outside the instrument under normal conditions.
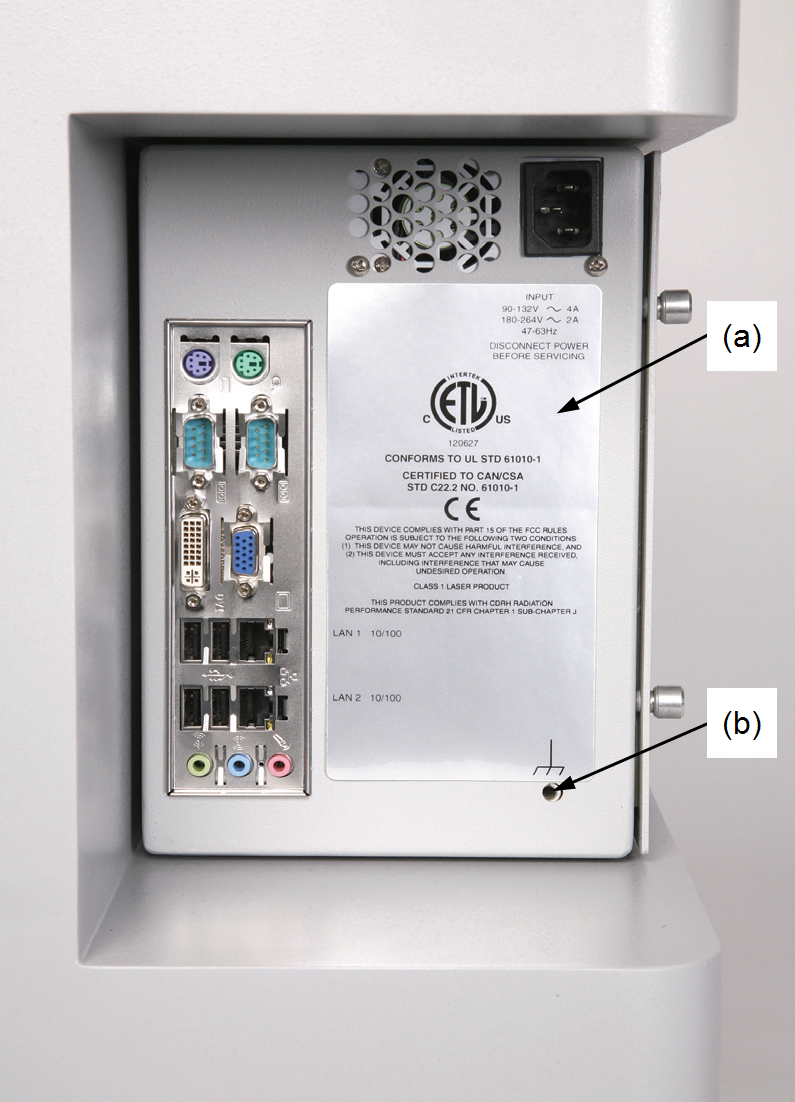
The CDRH implemented regulations for laser products on August 1, 1976 (CDRH radiation performance standard 21, Code of Federal Regulations Chapter 1, Sub-chapter J). Compliance for products marketed in the United States is mandatory. This product complies with CDRH radiation performance standard 21 CFR Chapter 1, Sub-chapter J. Ce produit est conforme à la performance de rayonnement CDRH chapitre CFR norme 1 Sous-chapitre J. The label that must be attached to laser products marketed in the United States is Figure 68 and is located on the rear panel of the Odyssey XF Imager (Figure 69), indicating compliance with CDRH regulations.
WARNING: Use of controls or adjustments or performance of procedures other than those specified herein may result in hazardous radiation exposure.
AVERTISSEMENT: L'utilisation des commandes ou le réglage ou la performance des procédures autrement qu'il a été indiqué dams le présent texte peut résulter en de dangereuses expositions aux radiations.
Safety Interlocks
Where there is a potential for class 4 laser radiation behind a given panel, the label in Figure 71 is affixed to the panel. The label shown in Figure 71 is affixed to the Odyssey XF in two locations; one on top of the camera hood and one on the inner front access panel (Figure 70).



The Odyssey® XF Imager has two safety interlocks that prevent exposure to laser radiation when the imaging drawer is opened during operation. Do not attempt to defeat these interlocks. The drawer interlocks are located in the back of the instrument.
Chemical Safety
LICORbio recommends that all biochemicals be handled carefully, and that safe laboratory procedures be followed at all times. Be aware of the hazards associated with any chemical before you begin work.
The LI‑COR® Odyssey® XF Imager should not be used with any radioactive materials.
Imaging Drawer Safety
Be careful not to get your fingers caught between the Odyssey XF Imager and the imaging drawer when the drawer closes. The drawer is specially designed to stop when a certain level of resistance is detected, so no harm should result, but the experience may be startling.
During an unexpected power loss, the drawer button will not function. This may leave a sample inside the Odyssey XF Imager, or it may result in the imaging drawer being left partially open or closed. If the power remains off, the drawer can be opened or closed by pulling or pushing on the drawer front. The imaging drawer should resume normal operation once power is restored.