Scanning Membranes
Scanning Membranes
For Western blotting methods, nitrocellulose or PVDF membranes may be used. LI‑COR offers Odyssey nitrocellulose membranes (10 membranes; 0.22 μm, 7 cm x 8.5 cm) under part number 926-31090. There are some general tips for using membranes:
- IMPORTANT: Do not touch the membrane – handle only with clean forceps. Lift the membrane only by the corners.
- Keep the membrane wet if it is to be stripped and re-used. This can easily be accomplished by wrapping the membrane in plastic wrap.
- Use clean containers to avoid cross-contamination and reduce background.
- Multiple membranes can be washed together, provided there is ample volume so each membrane moves freely.
Generally speaking, chemiluminescent detection protocols for Western blotting do not need to be modified for use with the C‑DiGit Blot Scanner. LI‑COR does, however, have a variety of resources available for optimizing Western blots. Some of the most common questions regarding imaging with the C‑DiGit Blot Scanner are listed below; more detailed support materials can be found at:
licor.com/bio/applications/chemiluminescent-western-blots
Placing the Membrane on the C‑DiGit Blot Scanner Scanning Surface
The imaging surface is designed to scan a single membrane up to 10 x 8.5 cm. Place the membrane face down, with the top of the membrane facing the back of the instrument.
Using Blocking Buffer
Can I use milk-based blockers?
Yes. Milk-based blockers can be used for chemiluminescent detection but should be avoided when detecting phosphoproteins or glycoproteins. Milk- based blockers may contain endogenous biotin and glycoproteins, resulting in higher background on the membrane.
Can I dilute the HRP-conjugated secondary antibodies in Odyssey® blocking buffer?
No. Odyssey blocking buffer contains sodium azide as a preservative. Sodium azide binds irreversibly to the HRP enzyme, inhibiting the binding of the substrate and slowing the chemiluminescent reaction. This results in less light production that may affect the appearance of less intense bands or even the entire blot. For optimal results do not use any solutions containing sodium azide for chemiluminescent Western blotting (for either secondary or primary antibodies).
What is the best blocker for chemiluminescent Western blots?
It is best to try several blockers to find the one that gives the most satisfying data for each antigen and antibody pair. There is not an optimum blocker for all conditions.
Primary and Secondary Antibodies
Why is the signal missing in the middle of the bands?
Too much secondary antibody on the membrane results in consumption of all of the substrate in that area. Without substrate, there is no chemiluminescent signal and a blank spot appears in the center of the band. Try different dilutions of the primary and secondary antibodies to find what gives the best results, or try changing the substrate.
Does it matter where I purchase the HRP-conjugated secondary antibodies?
The reactivity of secondary antibodies ranges widely between vendors. The ratio of HRP enzyme to antibody varies as well, and may affect the detection of the target. If the secondary antibodies from one vendor are not working, trying antibodies from LI‑COR or other vendors may be helpful. LI‑COR offers the WesternSure® Goat anti-Mouse HRP secondary antibody under part number 926-80010, and the WesternSure Goat anti-Rabbit HRP secondary antibody under part number 926-80011.
Should the HRP-conjugated secondary antibodies be highly cross- adsorbed?
Although highly cross-adsorbed antibodies are essential for two-channel, multiplex detection, it is not always necessary with chemiluminescent blotting for a single target.
Washing Buffer
Does it matter how I wash the membranes after antibody incubation?
Yes. Adequately washing the membranes will greatly improve the appearance of the chemiluminescent Western blot. Wash the membranes with a saline- buffered solution containing 0.05 to 0.1% of a non-ionic detergent such as Tween® 20. Wash four times for five minutes each on a shaker or rotator with ample wash solution.
Substrates
Which substrate should I use?
There are a wide variety of chemiluminescent substrates for HRP detection. In general, substrates with a faster reaction rate tend to be more sensitive. The C‑DiGit Blot Scanner is designed to work well with most substrates. LI‑COR carries a line of chemiluminescent substrates that have been optimized to work with the C‑DiGit Blot Scanner; two of these substrates are WesternSure ECL (p/n 926-80100) and WesternSure PREMIUM (p/n 926-95000).
How do I apply the substrate?
Make sure the substrate is at room temperature before use. Substrate should be applied by following the manufacturer’s suggested methods for the amount of substrate to use and the incubation time prior to imaging. The substrate can be directly added to the sample side of the membrane (either by pipetting the substrate on the sample surface, or by preincubating the membrane in the substrate), allowed to incubate, and scanned without having to wrap the membrane before scanning. Membranes can also be wrapped in a plastic covering prior to scanning.
How long should I incubate my blot?
Incubate the blot in substrate for 5 minutes before scanning. The incubation period is separate from the 6-minute acquisition time. Skipping or shortening the incubation may result in weak or no signal.
The membrane dried during imaging. Can I apply more substrate and image again?
No. Applying more substrate to a dried blot will likely result in high background.
How do I keep the membrane from drying out?
In general, the membrane should not dry out in the amount of time it takes to complete a scan. However, to maintain moisture on the membrane for an extended amount of time, place a clear, flat plastic covering on top of the chemiluminescent Western blot to keep the substrate in contact with the HRP enzyme and to prevent the blot from drying out. Membranes can also be completely wrapped in plastic covering prior to scanning (see Scanning Membranes). Make sure there is no plastic wrap extending out past the scanning surface and into the outer lid seal.
Imaging
Can I wrap the blot in plastic wrap before imaging?
As long as the plastic is clear, clean, and wrinkle free (the plastic wrap may cause unwanted background, especially if it is folded or handled roughly), the blot can be wrapped in plastic before imaging. Note that plastic wrap will cause unwanted background; we recommend using plastic wrap only if you must seal the membranes.
Why are the bands on my blot so light?
Use the Lookup Table (LUT) in Image Studio to adjust how data are mapped to the display pixels of your computer screen.
You can view the Image LUTs as either slider bars (the default view, below left) or as histograms overlaid with a curve (below right). To hide this view, click the double arrows in the top right corner.
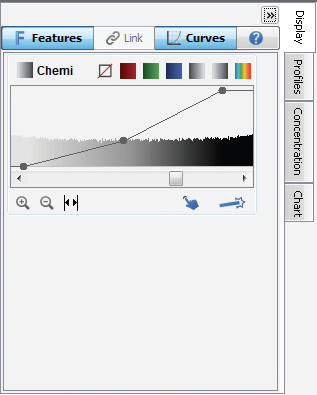
Figure 142. Overlaying the LUT histogram is a curve with three adjustable points (above right). Move the top Max Point to the left to map more of the higher intensity data to the brighter display pixels to make the bands appear brighter. Move the lower Min Point to the right to map the lower intensity data to the background color, creating a visually cleaner background. The middle point smoothly adjusts the mapping from linear to logarithmic. Changing to a more logarithmic mapping reduces the contrast between the lower and higher intensity data, so the appearance of less intense bands is improved while avoiding overly dark bands. 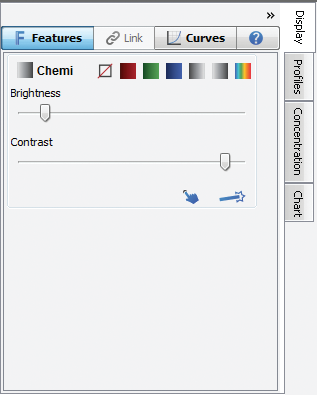
Figure 143. To change the Image LUTs from histograms (Curves) to Brightness/Contrast sliders, and vice versa, click on the Curves tab in the Image LUTs to toggle between histograms and Brightness/Contrast.
